Abstract
Introduction: Cancer cells elude anti-tumor immunity through multiple mechanisms, including upregulated expression of ligands for inhibitory immune checkpoint receptors. Blockade of signal regulatory protein (SIRP)-α on macrophages, or of its ligand CD47 expressed on tumor cells, improves tumor cell elimination however CD47 is a challenging target given its ubiquitous distribution in healthy tissues. Combination of CD47 blockade with the anti-CD20 antibody rituximab has resulted in encouraging clinical activity (Advani 2018), and CD19 is also an established target of multiple B-NHL therapies. TG-1801 is a bispecific agent designed with a CD19 high-affinity arm and a blocking CD47 arm for concurrent target engagement on the tumor cells surface. Herein we report results of the FIH study for the first time.
Methods: The primary objectives were to characterize the safety profile and to determine the recommended phase 2 dose (RP2D) of TG-1801. Other objectives included assessment of pharmacokinetics (PK), preliminary antitumor activity (Lugano 2014), and pharmacodynamics (PD: B-cell depletion). Eligible patients must have had relapsed/refractory (R/R) B-cell lymphoma after at least one prior standard therapy. A 3+3 design was utilized to evaluate sequentially higher doses of TG-1801 at the following dose levels: 20 mg, 60 mg, 180 mg, 360 mg, and 500 mg. In the monotherapy arm, treatment consisted of weekly fixed dosing 1h-infusions of TG-1801 in a 4-week cycle: day (D)1, D8, D15, and D22 of cycle (C) 1 and C2. Patients who had at least stable disease after 2 cycles were given the option of receiving TG-1801 once every 4 weeks for an additional 4 infusions. Intra-patient dose escalations were permitted. An additional arm was enrolled with the combination therapy of ublituximab (anti-CD20 mAb) plus TG-1801. Patients received TG-1801 and ublituximab infusions in a 4 week-cycle: D1, D8, and D15 of C1; D1 of C2 thru C6; and D1 of C9 and every 3 cycles thereafter, up to 24 cycles. A 3+3 design was also utilized to evaluate two dose levels of TG-1801 (300 mg and 400 mg) with a standard dose of ublituximab (900 mg).
Results: As of May 2022, 14 pts (MZL = 5, DLBCL including Richter's transformation = 5, FL = 4) received monotherapy and 16 pts received combination therapy (DLBCL = 9, FL = 4, MZL = 2, MCL = 1). Patients were heavily pretreated: median 3 prior systemic therapies (range, 1 - 8), all had received ≥1 prior anti-CD20; 14 pts (46%) were refractory to their last prior therapy. Twenty-one (70%) had extranodal disease, and 9 (30%) had bulky disease. One DLT of grade (G) 4 thrombocytopenia occurred at the 500 mg dose level. The most common (>20%) treatment emergent adverse events (TEAEs) at monotherapy doses of 20 mg to 360 mg (n=10) included fatigue, thrombocytopenia, and infusion-related reaction (3 pts each), with no G3/4 TEAE occurring in >20% of pts. Combination therapy was also well tolerated (TG-1801 at 300 mg N=3, 400 mg N=13) without DLT. The most common TEAE (≥20%) for combination therapy were: anemia (31%), headache, abdominal pain, fatigue, and thrombocytopenia (25% each), with no G3/4 TEAE occurring in >20% of pts. There were 2 permanent treatment discontinuations due to TEAE (1 infusion reaction [500 mg monotherapy], 1 rash [360 mg monotherapy]).
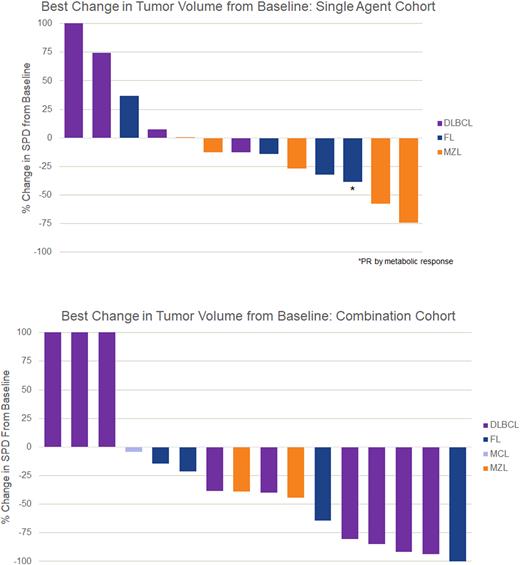
Three partial responses (PR) were observed on monotherapy (n=14), all at 360 mg TG-1801, including 1 MZL pt with 7 prior lines that included PI3Ki, BTKi, auto transplant, and multiple prior anti-CD20s. All 3 pts completed 6 cycles of treatment and continued to further cycles of re-treatment. In the combination arm (n=16), a 44% response rate was observed with 1 complete response (CR) in a pt with FL and 6 PR (5 pts with DLBCL and 1 pt with FL) for a 56% ORR for DLBCL patients and 50% ORR for FL patients. The median duration of combination therapy exposure was 8.7 mo (range of 1-20 mo). Responses appear durable, with ten pts remaining on study, with 1 pt receiving 360 mg monotherapy and 9 pts receiving combination therapy. Dose dependent increases in exposure were observed and pharmacodynamic analysis demonstrated a >50% depletion of circulating lymphocytes within 7 days of the first infusion in all evaluable pts.
Conclusions: TG-1801 monotherapy and combination therapy demonstrates clinical activity, particularly in relapsed and refractory DLBCL, with an acceptable preliminary safety profile. This study (NCT03804996) has completed enrollment.
Disclosures
Hawkes:Regeneron: Speakers Bureau; Specialised Therapeutics: Consultancy; Janssen: Speakers Bureau; Astra Zeneca: Speakers Bureau; Roche: Speakers Bureau; Roche: Research Funding; Bristol-Myers Squibb: Research Funding; Merck KgA: Research Funding; Astra Zeneca: Research Funding; Bristol_Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Antengene: Membership on an entity's Board of Directors or advisory committees; Link: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Merck Sharpe and Dohme: Membership on an entity's Board of Directors or advisory committees. Lewis:AstraZeneca: Consultancy, Honoraria; Janssen: Honoraria, Patents & Royalties; Loxo-Lilly: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; IQVIA: Membership on an entity's Board of Directors or advisory committees; Novartis: Patents & Royalties; Roche: Consultancy, Honoraria. Miskin:TG Therapeutics: Current Employment. Sportelli:TG Therapeutics: Current Employment. Kolibaba:TG Therapeutics, Inc: Consultancy, Current Employment; McKesson Specialty Heath: Membership on an entity's Board of Directors or advisory committees; Atara Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sunitomo Dainippon Oncology: Consultancy. Normant:TG Therapeutics, Inc: Current Employment. Turpuseema:TG Therapeutics, Inc: Current Employment. Cheah:Roche: Consultancy, Honoraria, Research Funding; Merck Sharp & Dohme: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Eli Lilly and Company: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal